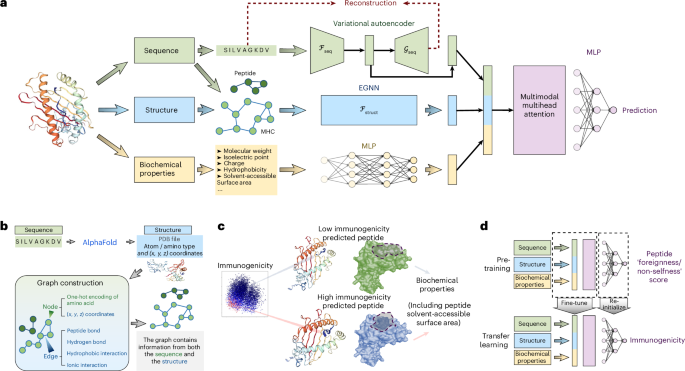
ImmunoStruct enables multimodal deep learning for immunogenicity prediction
Gupta, M. et al. Recent advances in cancer vaccines: challenges, achievements, and futuristic prospects. Vaccines 10, 2011 (2022).
Google Scholar
Fan, T. et al. Therapeutic cancer vaccines: advancements, challenges, and prospects. Signal Transduct. Target. Ther. 8, 450 (2023).
Google Scholar
Blass, E. & Ott, P. A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 18, 215–229 (2021).
Google Scholar
Bjerregaard, A.-M. et al. An analysis of natural T cell responses to predicted tumor neoepitopes. Front. Immunol. 8, 1566 (2017).
Google Scholar
Katsikis, P. D., Ishii, K. J. & Schliehe, C. Challenges in developing personalized neoantigen cancer vaccines. Nat. Rev. Immunol. 24, 213–227 (2024).
Google Scholar
Ott, P. A. et al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell 183, 347–362 (2020).
Google Scholar
Wells, D. K. et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 183, 818–834 (2020).
Google Scholar
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Google Scholar
Pishesha, N., Harmand, T. J. & Ploegh, H. L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 22, 751–764 (2022).
Google Scholar
Tynan, F. E. et al. The immunogenicity of a viral cytotoxic T cell epitope is controlled by its MHC-bound conformation. J. Exp. Med. 202, 1249–1260 (2005a).
Google Scholar
Wu, P. et al. Mechano-regulation of peptide-MHC class I conformations determines TCR antigen recognition. Mol. Cell 73, 1015–1027 (2019).
Google Scholar
Weber, J. K. et al. Unsupervised and supervised AI on molecular dynamics simulations reveals complex characteristics of HLA-a2-peptide immunogenicity. Brief. Bioinforma. 25, bbad504 (2024).
Google Scholar
Reynisson, B., Alvarez, B., Paul, S., Peters, B. & Nielsen, M. Netmhcpan-4.1 and netmhciipan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 48, W449–W454 (2020).
Google Scholar
Shao, X. M. et al. High-throughput prediction of MHC class I and II neoantigens with MHCnuggets. Cancer Immunol. Res. 8, 396–408 (2020).
Google Scholar
O’Donnell, T. J., Rubinsteyn, A. & Laserson, U. MHCflurry 2.0: improved pan-allele prediction of MHC class I-presented peptides by incorporating antigen processing. Cell Syst. 11, 42–48 (2020).
Google Scholar
Albert, B. A. et al. Deep neural networks predict class I major histocompatibility complex epitope presentation and transfer learn neoepitope immunogenicity. Nat. Mach. Intell. 5, 861–872 (2023).
Google Scholar
Saotome, K. et al. Structural analysis of cancer-relevant TCR-CD3 and peptide-MHC complexes by cryoEM. Nat. Commun. 14, 2401 (2023).
Google Scholar
Jiang, D. et al. Neoapred: a deep-learning framework for predicting immunogenic neoantigen based on surface and structural features of peptide–human leukocyte antigen complexes. Bioinformatics 40, btae547 (2024).
Google Scholar
Vita, R. et al. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 47, D339–D343 (2018).
Google Scholar
Koşaloğlu-Yalçın, Z. et al. The Cancer Epitope Database and Analysis Resource (CEDAR). Nucleic Acids Res. 51, D845–D852 (2023).
Google Scholar
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Google Scholar
Gfeller, D. et al. Improved predictions of antigen presentation and TCR recognition with MixMHCpred2. 2 and PRIME2.0 reveal potent SARS-CoV-2 CD8+ T-cell epitopes. Cell Syst. 14, 72–83 (2023).
Google Scholar
Kim, J. Y., Bang, H., Noh, S.-J. & Choi, J. K. DeepNeo: a webserver for predicting immunogenic neoantigens. Nucleic Acids Res. 51, W134–W140 (2023).
Google Scholar
Satorras, V. G., Hoogeboom, E. & Welling, M. E(n) equivariant graph neural networks. In Proc. International Conference on Machine Learning 9323–9332 (PMLR, 2021).
Kipf, T. N. & Welling, M. Semi-supervised classification with graph convolutional networks. In Proc. 5th International Conference on Learning Representations (ICLR, 2017).
Vaswani, A. et al. Attention is all you need. In Proc. Advances in Neural Information Processing Systems 5998–6008 (NIPS, 2017).
Xiao, X. et al. HGTDP-DTA: hybrid graph-transformer with dynamic prompt for drug-target binding affinity prediction. In Proc. International Conference on Neural Information Processing 340–354 (Springer, 2024).
Kingma, D. P. Auto-encoding Variational Bayes. In Proc. International Conference on Learning Representations (ICLR, 2014).
Liao, D. et al. RNAGenScape: property-guided optimization and interpolation of mRNA sequences with manifold Langevin dynamics. Preprint at https://doi.org/10.48550/arXiv.2510.24736 (2025).
Liu, C. et al. DiffKillR: killing and recreating diffeomorphisms for cell annotation in dense microscopy images. In Proc. IEEE International Conference on Acoustics, Speech and Signal Processing 1–5 (IEEE, 2025).
Sun, X. et al. Geometry-aware generative autoencoders for warped Riemannian metric learning and generative modeling on data manifolds. In Proc. International Conference on Artificial Intelligence and Statistics (PMLR, 2024).
Osorio, D., Rondón-Villarreal, P. & Torres, R. Peptides: a package for data mining of antimicrobial peptides. R J. 7, 4–14 (2015).
Zhuang, F. et al. A comprehensive survey on transfer learning. Proc. IEEE 109, 43–76 (2020).
Google Scholar
Richman, L. P., Vonderheide, R. H. & Rech, A. J. Neoantigen dissimilarity to the self-proteome predicts immunogenicity and response to immune checkpoint blockade. Cell Syst. 9, 375–382 (2019).
Google Scholar
Łuksza, M. et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520 (2017).
Google Scholar
Balachandran, V. P. et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 (2017).
Google Scholar
Perry, J. S. A. & Hsieh, C.-S. Development of T-cell tolerance utilizes both cell-autonomous and cooperative presentation of self-antigen. Immunol. Rev. 271, 141–155 (2016).
Google Scholar
Ghorani, E. et al. Differential binding affinity of mutated peptides for MHC class I is a predictor of survival in advanced lung cancer and melanoma. Ann. Oncol. 29, 271–279 (2018).
Google Scholar
Moon, K. R. et al. Visualizing structure and transitions in high-dimensional biological data. Nat. Biotechnol. 37, 1482–1492 (2019).
Google Scholar
Tadros, D. M., Eggenschwiler, S., Racle, J. & Gfeller, D. The MHC motif atlas: a database of MHC binding specificities and ligands. Nucleic Acids Res. 51, D428–D437 (2023).
Google Scholar
Sidney, J., Peters, B., Frahm, N., Brander, C. & Sette, A. HLA class I supertypes: a revised and updated classification. BMC Immunol. 9, 1 (2008).
Google Scholar
Nguyen, A. T., Szeto, C. & Gras, S. The pockets guide to HLA class I molecules. Biochem. Soc. Trans. 49, 2319–2331 (2021).
Google Scholar
Tynan, F. E. et al. T cell receptor recognition of a ‘super-bulged’ major histocompatibility complex class I-bound peptide. Nat. Immunol. 6, 1114–1122 (2005b).
Google Scholar
Tynan, F. E. et al. At cell receptor flattens a bulged antigenic peptide presented by a major histocompatibility complex class I molecule. Nat. Immunol. 8, 268–276 (2007).
Google Scholar
Ding, Y.-H. et al. Two human T cell receptors bind in a similar diagonal mode to the HLA-a2/tax peptide complex using different TCR amino acids. Immunity 8, 403–411 (1998).
Google Scholar
Borch, A. et al. Improve: a feature model to predict neoepitope immunogenicity through broad-scale validation of T-cell recognition. Front. Immunol. 15, 1360281 (2024).
Google Scholar
Rappaport, A. R. et al. A shared neoantigen vaccine combined with immune checkpoint blockade for advanced metastatic solid tumors: phase 1 trial interim results. Nat. Med. 30, 1013–1022 (2024).
Google Scholar
Chen, J.-L. et al. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J. Exp. Med. 201, 1243–1255 (2005).
Google Scholar
Lu, D. et al. KRAS G12V neoantigen specific T cell receptor for adoptive T cell therapy against tumors. Nat. Commun. 14, 6389 (2023).
Google Scholar
Poole, A. et al. Therapeutic high affinity T cell receptor targeting a KRASG12D cancer neoantigen. Nat. Commun. 13, 5333 (2022).
Google Scholar
Nusrat, F. et al. The clinical implications of KRAS mutations and variant allele frequencies in pancreatic ductal adenocarcinoma. J. Clin. Med. 13, 2103 (2024).
Google Scholar
Weiskopf, D. et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl Acad. Sci. USA 110, E2046–E2053 (2013).
Google Scholar
Hinton, G. E. & Salakhutdinov, R. R. Reducing the dimensionality of data with neural networks. Science 313, 504–507 (2006).
Google Scholar
Liu, C. et al. Imageflownet: forecasting multiscale trajectories of disease progression with irregularly-sampled longitudinal medical images. In Proc. IEEE International Conference on Acoustics, Speech and Signal Processing 1–5 (IEEE, 2025).
Liu, C. et al. Cuts: a deep learning and topological framework for multigranular unsupervised medical image segmentation. In Proc. International Conference on Medical Image Computing and Computer-Assisted Intervention 155–165 (Springer, 2024).
Paszke, A. et al. PyTorch: an imperative style, high-performance deep learning library. In Proc. Advances in Neural Information Processing Systems 32, 8026–8037 (NIPS, 2019).
Chen, T., Kornblith, S., Norouzi, M. & Hinton, G. A simple framework for contrastive learning of visual representations. In Proc. International Conference on Machine Learning 1597–1607 (PMLR, 2020).
Zbontar, J., Jing, L., Misra, I., LeCun, Y. & Deny, S. Barlow twins: self-supervised learning via redundancy reduction. In Proc. International Conference on Machine Learning 12310–12320 (PMLR, 2021).
Loshchilov, I. & Hutter, F. Decoupled weight decay regularization. In Proc. International Conference on Learning Representations (ICLR, 2019).
Loshchilov, I. & Hutter, F. SGDR: stochastic gradient descent with warm restarts. In Proc. International Conference on Learning Representations (ICLR, 2017).
Sette, A. & Sidney, J. Nine major HLA class I supertypes account for the vast preponderance of HLA-a and -b polymorphism. Immunogenetics 50, 201–212 (1999).
Google Scholar
Mirdita, M. et al. Colabfold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Google Scholar
Jamasb, A. et al. Graphein—a Python library for geometric deep learning and network analysis on biomolecular structures and interaction networks. In Proc. Advances in Neural Information Processing Systems 35, 27153–27167 (NIPS, 2022).
Zaidi, N. et al. Role of in silico structural modeling in predicting immunogenic neoepitopes for cancer vaccine development. JCI Insight 5, (2020).
Slota, M., Lim, J.-B., Dang, Y. & Disis, M. L. ELISpot for measuring human immune responses to vaccines. Expert Rev. Vaccines 10, 299–306 (2011).
Google Scholar
Yang, F. et al. Validation of an IFN-gamma ELISpot assay to measure cellular immune responses against viral antigens in non-human primates. Gene Ther. 29, 41–54 (2022).
Google Scholar
Nelde, A. et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 22, 74–85 (2021).
Google Scholar
Givenchian, K. B. et al. ImmunoStruct: ImmunoStruct release. Zenodo https://doi.org/10.5281/zenodo.17535443 (2025).
Don’t miss more hot News like this! Click here to discover the latest in AI news!
2025-12-31 00:00:00




