FDA Approves First At-Home Test for Cervical Cancer Screenings
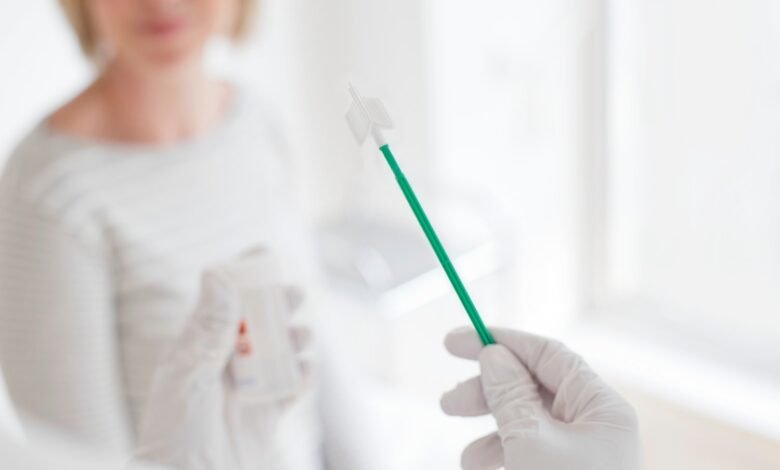
PAP staining is a vital tool for preventing cervical cancer because it can discover cells before it becomes cancerous. Unfortunately, systematic barriers or discomfort with the procedure means that many people do not receive them. But on Friday, the food and Drug Administration agreed to the first home test to display cervical cancer, which is a lesser alternative and perhaps easier in the cervical surfaces.
The newly approved test was developed by the FDA by San Francisco. Like traditional cervical swabs, wild ducks test for HPV or HPV, which causes almost all cervical cancers. However, it is the test, the wild duck stick, only requires a vaginal tin with a sponge -like tool rather than inserting a mitigation of the cervical climbing of the cervix.
In a statement, Kara Aegean, CEO and co-founder of Teal Health, said that the approval is not “just an innovative product-it finally related to giving women a logical choice for their lives-something that can be done quickly and comfortably at home.”
“Because when we make care easier to get it, we help women stay in good health, and for the people who depend on them every day,” Ighan continued.
In the United States, about 11,500 new cases of cervical cancer are diagnosed every year, and 4000 people will die of it. However, cervical cancer can be “eliminating[d] “In our lives,” Dr. Alexei Wright, Oncology Research Director at the Dana Farper Cancer Institute, told the New York Times. We not only have a comprehensive HPV vaccination or cervical cancer examination, and we often cannot reach people at a higher risk. “
According to the American Cancer Society, cervical cancer deaths in the United States decreased by more than half since the mid -seventies of the twentieth century, although it was recently connected. One study found 2022 that 23 % of people were behind their shows in 2019 compared to 14 % in 2005. In addition, cervical cancer rates are 25 % higher in rural areas, 42 % higher death rate, most likely to resource lack and health care providers. For some, discomfort is also a large barrier. In 2023, many participants in the Michigan medicine study said that groups at home “provide a comfortable, comfortable and flexible option without shock.”
The shows at home are effective alternatives to close the examination gap. One study found that postal groups of the late people of cervical cancer shows witnessed an increase of a 50 % increase compared to the provision of usual care alone. In addition, it is accurate like clinical tests. (Teal Health also has a self -maid study.)
Tail Health plans to provide sets next month, starting with California. The company also coordinates with the main insurance providers in the hope of covering costs. In addition to requesting tests through the Teal Health platform, people can coordinate follow -up care through it if necessary.
Don’t miss more hot News like this! Click here to discover the latest in Technology news!
2025-05-11 14:50:00